electron withdrawing groups|electron donating effect : Clark Electron-withdrawing groups are the opposite effect of electron-donating groups (EDGs). Both describe functional groups, however, electron-withdrawing groups pull electron density away from a molecule, whereas EDGs push electron density onto a substituent. Enjoy Pure Taboo porn videos for free. Watch high quality HD Pure Taboo tube videos & sex trailers. No password is required to watch movies on Pornhub.com. The most hardcore XXX movies await you here on the world's biggest porn tube so browse the amazing selection of hot Pure Taboo sex videos now.
PH0 · inductive electron withdrawal
PH1 · electron withdrawing inductive effect
PH2 · electron withdrawing groups list
PH3 · electron withdrawing effect
PH4 · electron donating effect
PH5 · electron donating and withdrawing groups
PH6 · electron donating ability
PH7 · diels alder stereochemistry
PH8 · Iba pa
Daylight Saving: This is a standard time zone, however during summer some places switch clocks for one hour forward when daylight saving comes into effect and observe Pacific Daylight Time (PDT). End: Pacific Standard Time (PST) has ended on Sunday, March 10, 2024 at 2:00 am local time and clocks were set one hour forward to Sunday, March 10, .
electron withdrawing groups*******The more electron-rich the aromatic ring, the faster the reaction. Groups that can donate electron density to the ring make EAS reactions faster. If a substituent increases the rate of reaction relative to H it is called activating. If it decreases the rate relative to H .
Electron-withdrawing groups are the opposite effect of electron-donating groups (EDGs). Both describe functional groups, however, electron-withdrawing groups pull electron density away from a molecule, whereas EDGs push electron density onto a substituent. Electron donating groups are alkyl groups, phenyl groups or substituents that have a lone pair of electrons on the atom directly bonded to the ring. Electron .Because Lewis acid-base reactions involve electron donation and acceptance at particular sites, substituent groups which alter the electron density at a site through inductively donating or withdrawing electron .
electron donating effectBecause Lewis acid-base reactions involve electron donation and acceptance at particular sites, substituent groups which alter the electron density at a site through inductively donating or withdrawing electron .Carbonyl groups are electron-withdrawing by inductive effects, due to the polarity of the C=O double bond. It is possible to demonstrate in the laboratory that carbocation A below is more stable than carbocation B, .
The web page you requested is not available due to a glitch. It is supposed to explain substituent effects in electrophilic substitutions, including electron withdrawing groups. Additional mechanistic and computational studies indicate that the key phenoxyl intermediate serves as an open-shell electron-withdrawing group in these reactions, lowering the barrier for . Each molecule was divided into π-spacer (chrysene) and benzothiophene acceptor units with diverse electron-withdrawing groups (refer to Fig. 1). Almost, all the derivatives show a similar pattern . Finally, substrates without an electron-donating group or with electron-withdrawing functionalities on the aromatic core showed higher oxidation potentials at . For this reason, unequivocal identification of structural factors determining electron donating/withdrawing properties of specific groups attached to the .
As described earlier in this section, hydroxyl, alkoxyl, and amino groups have a strong, electron-donating resonance effect that outweighs a weaker electron-withdrawing inductive effect. When phenol is nitrated, for instance, reaction can occur either ortho, meta, or para to the –OH group, giving the carbocation intermediates shown in Figure .
Avoid misconceptions about the impact of\(\pi\) donation and acceptance on Lewis acid-base affinity; References: The conjugate bases of many Brønsted superacids have electron-withdrawing substituents that .
Here are some general pointers for recognising the substituent effects: The H atom is the standard and is regarded as having no effect. Activating groups increase the rate. Deactivating groups decrease the rate. EDG . The presence of an electron-withdrawing group - such as a fluorine atom - will significantly destabilize a carbocation through the inductive effect. Carbonyl groups are electron-withdrawing by inductive effects, due to the polarity of the \(C=O\) double bond. It is possible to demonstrate in the laboratory (we'll see how in problem 14.x) that .Electron withdrawing group (EWG): An atom or group that draws electron density from neighboring atoms towards itself, usually by resonance or inductive effects. Trifluoro acetate ion is a weaker base than acetate ion because the trifluoromethyl group is attracting electron density away from the carboxylate .
Electron withdrawing group (EWG): An atom or group that draws electron density from neighboring atoms towards itself, usually by resonance or inductive effects. Trifluoro acetate ion is a weaker base than acetate ion because the trifluoromethyl group is attracting electron density away from the carboxylate .Just as electron-donating groups can stabilize a carbocation, electron-withdrawing groups act to destabilize carbocations. Carbonyl groups are electron-withdrawing by inductive effects, due to the polarity of the C=O double bond. It is possible to demonstrate in the laboratory that carbocation A below is more stable than carbocation B, even . While heteroatom-centered radicals are understood to be highly electrophilic, their ability to serve as transient electron-withdrawing groups and facilitate polar reactions at distal sites has not been extensively developed. Here, we report a new strategy for the electronic activation of halophenols, wherein generation of a phenoxyl .
Electron with-drawing groups can decrease the electron density at the nucleus, deshielding the nucleus and result in a larger chemical shift. Compare the data in the table below. . The effects are cumulative so the presence of more electron withdrawing groups will produce a greater deshielding and therefore a larger chemical shift, i.e .
EWG, electron-withdrawing group. Full size image. Radical philicity and HAT chemistry. Radical philicity can influence the outcome of many types of organic reactions, .
Electron withdrawing group (EWG): An atom or group that draws electron density from neighboring atoms towards itself, usually by resonance or inductive effects. Trifluoro acetate ion is a weaker base than acetate ion because the trifluoromethyl group is attracting electron density away from the carboxylate .
The nitro group (-NO 2), and the positively charged, tetra-substituted amino group (consider the structure once this trimethyl amino group is connected to the aryl ring) are both electron-withdrawing. As the trimethyl amino group will have an overall positive charge (and the nitro group is neutral overall), the trimethyl amino group is the . Electron donating groups usually have lone pairs of electrons that can get mingled with the 6 pi electrons & take part in resonance. Electron withdrawing groups usually have an atom .The participation of the electron-withdrawing group in π-conjugation decreases the LUMO level and narrows the energy gap in the order of perfluoroalkyl, acyl, perfluoroacyl, nitro ≈ dicyanovinyl in both series. TD-DFT calculations allowed better understanding of electronic transitions.An electron withdrawing group or EWG draws electrons away from a reaction center. When this center is an electron rich carbanion or an alkoxide anion, the presence of the electron-withdrawing substituent has a stabilizing effect. Examples of electron withdrawing groups are

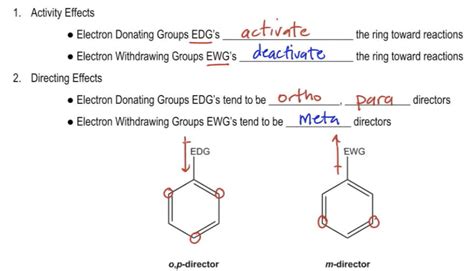
And so a substituent that overall decreased the electron density in the ring, and we could say it's an electron withdrawing group because it's withdrawing electron density .electron withdrawing groups And so a substituent that overall decreased the electron density in the ring, and we could say it's an electron withdrawing group because it's withdrawing electron density . In the previous episode we discussed what happens when we use electrophilic aromatic substitution to add a group to a benzene ring, but what happens when you.electron withdrawing groups electron donating effectOkay, Whereas many directors electron withdrawing groups, right, they pull electrons out of the ring, so they're gonna tend to add in the meta positions. Okay, I forgot to draw the Dye poll of the electron donating is gonna push electrons into the ring. It directs towards Opie, whereas electron withdrawing groups direct towards the meta positions.
Loto Results Lotto Lebanon - Zeed Results 2244. Loto Libanais Zeed draws every Mondays and Thursdays at 7:30PM. Here are Zeed winning numbers for the draw #2244
electron withdrawing groups|electron donating effect